BACKGROUND : Hematopoietic stem cell transplantation (HSCT) is broadly utilized for the treatment of lymphoma. Autologous peripheral blood stem cell (PBSC) transplantation (ASCT) requires mobilization of hematopoietic stem cell/ hematopoietic progenitor cell (HSC/HPC) in peripheral blood (PB) to collect HSCs. The high mobilization failure rate with the mobilization strategy of combining chemotherapy and filgrastim (rhG-CSF, recombinant human granulocyte colony-stimulating factor) in ASCT is one of the unresolved issues. Whether the combination of polyethylene glycol filgrastim [pegfilgrastim (PEG-FIL), PEG-rhG-CSF] and filgrastim (FIL) improves the mobilization success rate and the timing of combination therapy has not been studied.
METHODS: To test the hypothesis that the combination of pegfilgrastim and filgrastim schedule has superiority, we retrospectively investigated a cohort of 107 lymphoma patients who received auto-HSCT and collection in the First Hospital of Jilin University from Jan 1st 2015 to Dec 1st 2022. These patients were categorized into PEG+FIL group and FIL group. The group of PEG+FIL received pegfilgrastim (9 mg) on the third day of the chemotherapy, followed by filgrastim (10 μg/kg/day) based on the counts of peripheral blood stem cells (PBSC). The group of FIL received filgrastim 10 μg /kg/day depending on the number PBSCs. Demographic information, laboratory results, and treatment plans were collected for these two groups of patients.
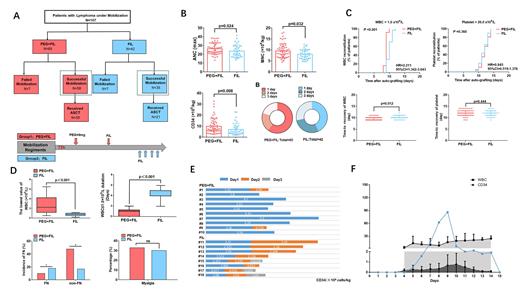
RESULTS:We compared their mobilization efficacy (mononuclear cell [MNC] and CD34 + cell counts) and transplantation efficacy (days to leukocyte and platelet recovery post-transplantation). Successful ASCT mobilization was achieved in 93 patients (86.9%), with the PEG+FIL group exhibiting a higher success rate compared to the FIL group (89.2% [58/65] vs. 83.3% [35/42], p = 0.032). The PEG+FIL group also demonstrated significantly higher CD34+ cell counts [MNC: 8.9 (6.9, 11.2) vs. 7.8 (6.2, 9.8) × 10 8 cells/kg, p = 0.024; CD34 + cell count: 7.5 (5.6, 13.0) vs. 4.9 (3.2, 9.2) × 10 6 cells/kg, p = 0.008] and a lower incidence of neutropenic fever compared to the FIL group (11.8% vs. 24.3%, p = 0.038). The PEG+FIL group also demonstrated a shorter mean leukocyte recovery time in autologous stem cell transplantation than the FIL group (10.02 ± 0.73 vs. 10.9 ± 1.1 days, p < 0.001).
To delve deeper into the safety considerations of both groups, we evaluated the advantages of each regimen and the methodologies for selecting time windows. A bone marrow aspiration and biopsy were performed on all patients before stem cell collection. Among 18 patients who had a hematopoietic area less than 30% and a fat area greater than 40%, 10 were in the PEG+FIL group and 8 in the FIL group. For patients who required only one collection, the durability was 80% in the PEG+FIL group compared to 0% in the FIL group (p < 0.01), indicating a superior recommendation for patients with a bone marrow hematopoietic area of less than 30%. In the PEG+FIL group, monitoring was carried out for PB CD34 + percentage and WBC count following pegfilgrastim administration, with subsequent computation of CD34 + cells in PB. Filgrastim administration was found to be optimal 5-6 days post-pegfilgrastim administration.
CONCLUSIONS: In conclusion, relative to conventional filgrastim mobilization, the conjunctive use of pegfilgrastim and filgrastim demonstrates superior efficacy, non-inferior safety during ASCT. The schedule enhanced the the outcomes of mobilization in the patients of less hematopoietic area.
KEY WORDS: Pegylated recombinant human granulocyte-stimulating factor (pegfilgrastim, PEG-rhG-CSF), recombinant human granulocyte-colony stimulating factor (filgrastim, rhG-CSF), lymphoma, hematopoietic stem cell transplantation (HSCT) autologous hematopoietic stem cell mobilization.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal